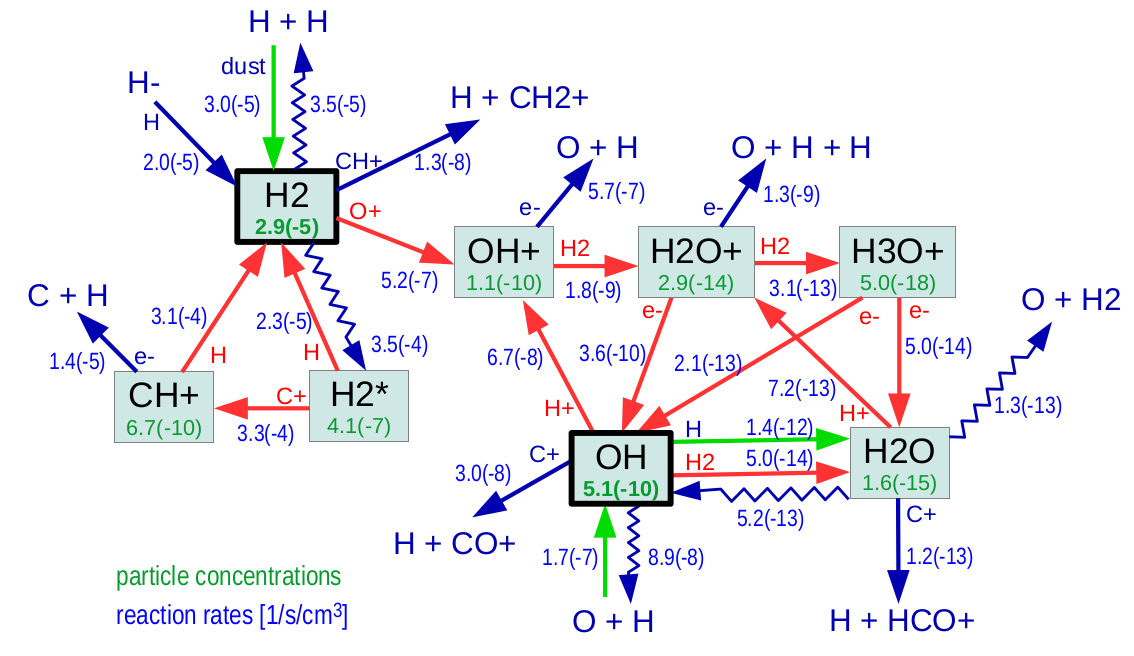
With ProDiMo you can use various standard chemical rate networks (KIDA, UMIST, OSU) in combination with your own selection of species and reaction rates. ProDiMo comes with an analysis mode where you can study how certain molecules are formed at a selected position in the disk. The figure shows how H2, OH, and H2O are formed at r=10 au at vertical column desity NH ~ 10+20 cm-2. Here we have a hydrogen nuclei density of n<H>=2 x 10+7cm-3. The gas and dust temperatures are 347 K and 126 K and the point is optically thin with radial and vertical visual extinctions Av_rad = 0.024 and Av_ver = 6.3 x 10-4, respectively.
The blue numbers show the rates [cm-3s-1] and the green numbers show the concentrations ni/n<H>. Wiggled arrows indicate photo-dissociation. H2* means electronic excited molecular hydrogen. Light green arrows mark the H2-formation on grains and the radiative association reactions O + H -> OH + hv and OH + H -> H2O + hv. The reaction cycles are completed by photo-dissociation and dissociative recombination reactions, which again destroy the molecules and replenish the atoms.
